CUSTOMER SUCCESS STORY
University of Vienna: Orders of Magnitude Improvements in Radioisotope Analysis
The Challenge
Many areas of environmental science, geology, astrophysics (meteorites), archeology, and other fields rely on the use of long-lived radioisotopes – radioactive nuclides with long half-lives – to determine the sample’s age, source, and other historical imprints. The figure of merit here is the isotope ratio: the concentration of the radioisotope vs. stable isotopes of the same element. The most famous of course is carbon-14 (14C) that is widely used in “carbon dating.” Unfortunately, these useful radioactive markers are typically only present in extremely low concentrations. The most sensitive way to measure their ultra-low concentrations is called Accelerator Mass Spectrometry (AMS) which can sometimes reach attomolar sensitivity. It also requires only milligrams of sample vs alternative methods based on radiation detection. However, a major limitation of AMS is the presence of unwanted isobars that can completely overwhelm the signal of some target nuclides.
AMS is a multistep process that is designed to create an accelerated (up to several MeV) beam of first negatively and then positively charged ions that are discriminated multiple times by deflection using strong magnetic fields, where the deflection depends on the mass/charge (m/q) ratio. Upstream of the acceleration, the sample is first bombarded to produce a beam of negative ions (anions) that, following m/q-analysis, are injected into the accelerator, where they are converted into positive ions (cations) in a device called a stripper that also eliminates small molecular ions that might have the same m/q ratio as the target nuclide, e.g., 14C- and 12CH2-. The cations are then further accelerated and again sorted according to their m/q ratios. But for many radioisotopes there is still the problem of isobars: isotopes of different elements with the same approximate mass which can outnumber the radioisotope of interest (e.g., 135Cs and the isobar 135Ba) by several orders of magnitude. The only conventional way to address the isobar problem has been to use extremely high (>10 MV) voltage acceleration to sort the ions based on their tiny nuclear charge details. This limited the measurement capability to only a few facilities around the world and a handful of radioisotopes.
Fortunately, a team including Dr. Martin Martschini in the physics faculty at the University of Vienna have developed an elegant and powerful alternative based on the clever use of lasers to perform AMS in conjunction with selective photodetachment.
The Solution
Their AMS system is called the Vienna Environmental Research Accelerator (VERA). The key to effectively eliminating isobar background signals in VERA is their recent development of Ion Laser InterAction Mass Spectrometry (ILIAMS). This is a new kind of element-selective filter and is the first device of its kind worldwide coupled to an AMS-facility.
ILIAMS is implemented at the anion stage (the “low energy mass spectrometer”) and works by threshold photodetachment. Martschini states, “Molecular and atomic anions all have a threshold photon energy for photodetachment of the extra electron; absorption of photons above this energy leads to ejection of an electron, i.e., neutralization of the anion. For each radionuclide of interest, we carefully design the chemical preparation of the sample so that the anion containing the radionuclide is one with a higher threshold for this ionization than the anion(s) of the problem isobar. We then select a laser wavelength with a photon energy above the threshold for the isobar ions but below that for that target radionuclide.” The laser thus eliminates problem isobars from the ion beam before the stripper and acceleration stage. In theory, all that is required is a laser somewhere in the appropriate wavelength (photon energy) window. So to enable VERA to perform research and to provide measurement services based on different radioisotopes and their isobar backgrounds, a portfolio of several different laser wavelengths is required.
However, the laser requirements go beyond just matching a fairly wide wavelength window. That’s because a challenge with ILIAMS is the low cross-section (probability) for the laser absorption and photodetachment mechanism. Martschini explains, “To counteract this we need high laser intensity and to extend the laser/anion interaction time to a millisecond or more.” They accomplish the latter by having the anion beam pass through a radiofrequency (RF) ion-guide filled with an inert buffer gas (typically He). This slows the anions almost down to thermal energies. The ion-guide is 1 meter long and providing the laser beam is exactly colinear with the anion beam in this ion-guide, then the laser/ion interaction time is about1 ms, which enables effective isobar elimination.
Two of the lasers at VERA are the Coherent AVIA LX at 355 nm and the Verdi 18 producing up to 20 watts at 532 nm. Martschini states, “We chose these lasers for several reasons. First, they offer the requisite power for ILIAMS to be very effective. Second, they have circular TEM00 output beams with M2 fairly close to 1.0. This high beam quality is critical to our technique since we have to align the beam through 3 mm apertures at each end of the 1 meter ion-guide beam path, which sits 3 meters downstream from the entry window into the vacuum system and the last optical lens or mirror. And lastly, they are very reliable. Each data run takes 1-6 hours. But we typically schedule a week of experiments targeting the same specific isotope and isobar. We need perfect laser performance for that entire week every time we schedule its wavelength, or we run into major scheduling issues.”
The Result
When ILIAMS was first under development, the team hoped it would be able to remove isobar backgrounds by as much as 6-8 orders of magnitude, but it has proved even better than that lofty goal for some isobars. According to Martschini, “A standout example is the separation of sulfur and chlorine, where we are able to suppress 36S relative to 36Cl, by an incredible 11 orders of magnitude using the 532 nm output from the Verdi laser.”
Martschini summarizes that thanks to ILIAMS, more and more new trace isotopes have become accessible to AMS for the first time at environmental levels. Recent additions include the long-lived fission product 90Sr (highest sensitivity of any detection technique worldwide). Other great examples he cites include the cesium radioisotopes 135Cs and 137Cs, which were both previously notoriously difficult targets. Specifically, with traditional AMS, it was nearly impossible to count 135Cs and 137Cs ions from some environmental samples like seawater, because barium isotopes of the same nominal mass can be a million times (or more) abundant. But these are environmentally important radioisotopes that are present in the ocean and fish due to nuclear testing in the 1950s and 1960s, and more recently due to the Fukushima nuclear disaster. Fortunately, their measurement is now relatively straightforward even at ultra-trace levels thanks to ILIAMS.
Meanwhile, the quest to determine protocols and wavelengths to other radioisotopes continues unabated at VERA. Looking to the future Martschini states that the ILIAMS team are currently working hard to add the astrophysically interesting 182Hf.
Lasers have long been used for analytical purposes – to interrogate samples according to their composition, phase, temperature, and so forth. But the innovative ILIAMS method is enabling scientists at the University of Vienna and their collaborators around the world to use lasers to investigate the detailed history of all kinds of samples as never before.
“The high beam quality of the AVIA LX as well as the VERDI laser is critical to our technique since we have to align the beam through 3 mm apertures at each end of the 1-meter waveguide beam path."
— Martin Martschini, Senior Scientist, Isotope Physics Group, University of Vienna


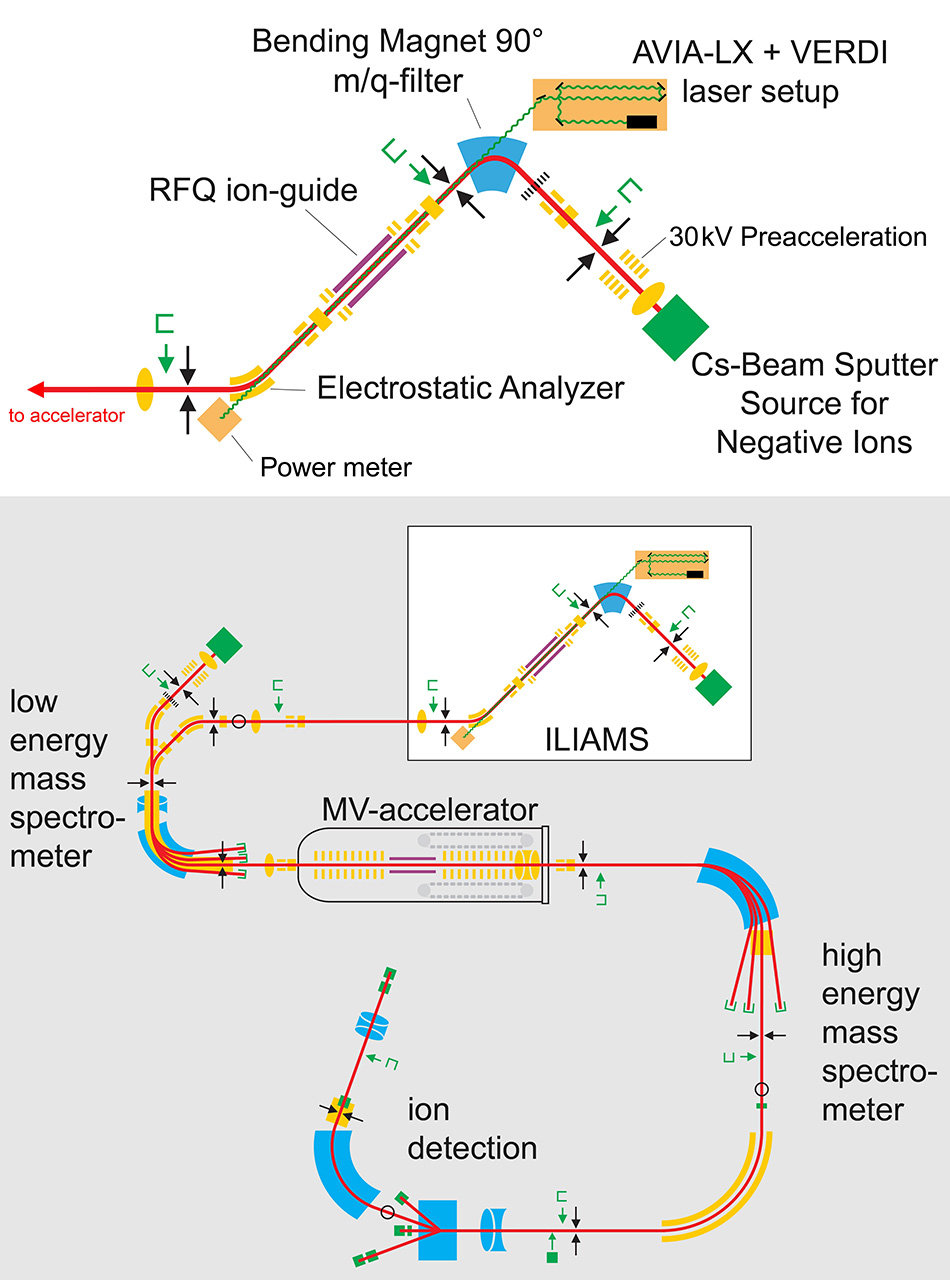
Figure 1. In the ILIAMS setup, the initial beam of anions is cooled in a waveguide while being irradiated by a colinear laser beam of the appropriate wavelength to cause selective electron photodetachment. Figure credit Martin Martschini.
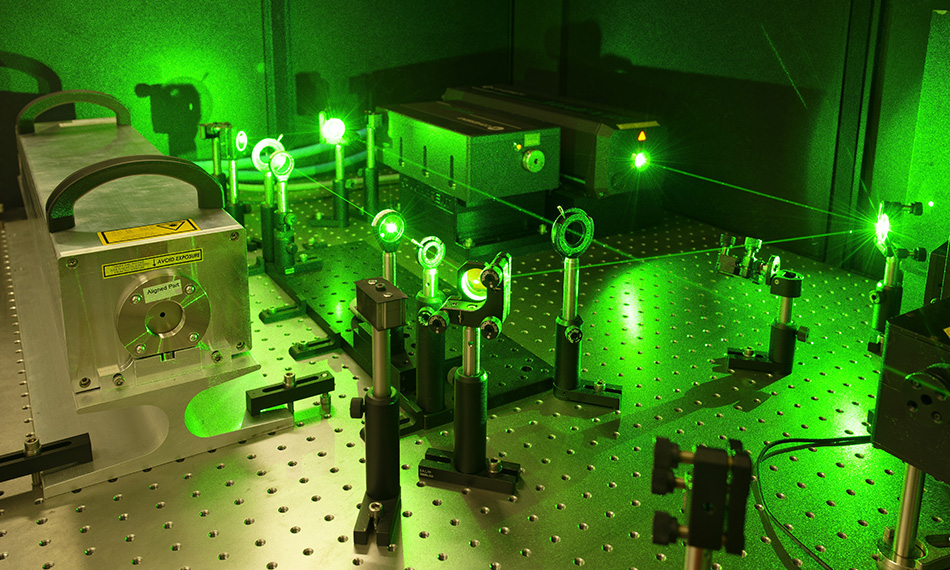
Figure 2. Together with the ultraviolet AVIA LX, the Verdi 18 is a key laser in the ILIAMS setup, enabling unwanted background signals from isobars to be suppressed by up to 11 orders of magnitude. Figure credit Martin Martschini.