SOLUTION BRIEF
Polymorph Identification
in Pharmaceuticals
Introduction
Many active pharmaceutical ingredients (APIs) exhibit polymorphism, where different forms or molecular structures of a compound can dramatically affect the efficacy, stability and bio-availability of the drug. These structural changes may occur during various stages of formulation, storage, packaging and handling. Consequently, rapid and reliable identification of polymorphs during the development, manufacturing, and quality assurance process is critical in pharmaceutical manufacturing.
Traditional Solutions
Observing structural shifts of a compound can be accomplished several ways. Raman spectroscopy is used to observe small band shifts in the “fingerprint” region (200-1800 cm-1), however these reflect subtle shifts in functional groups and are difficult to detect for some polymorphs. X-ray diffraction (XRD) techniques yield extremely quantitative and conclusive analysis, but require expensive equipment and destructive off-line testing. Terahertz (THz) spectroscopy can easily differentiate structural shifts, as these signals correspond to large scale motions in the molecular and inter-molecular structure, however THz spectroscopy has limited spectroscopic range, is expensive, and can require special sample preparation.
Coherent Solutions
Coherent THz-Raman® systems extend the range of traditional Raman spectroscopy to the terahertz/low frequency regime, where differentiation of inter- and intra-molecular structures can be clearly seen. THz-Raman spectra can also be used to differentiate raw materials, synthetic pathways, and contaminants, useful for counterfeit detection and surety testing. Anti-Stokes signals add to Raman intensity and improve SNR. Coherent THz-Raman® systems provide fast, unambiguous differentiation of polymorphs, while preserving the complete Raman “fingerprint region” for chemical identification.
Application Field
Polymorph Identification and Screening using low-frequency THz-Raman Spectroscopy.
* Data courtesy Dr. Tatsuo Koide, National Institute of Health Sciences, Division of Drugs, Tokyo, Japan.

Figure 1: THz-Raman delivers improved senstivity and realiability for screening of active pharmaceutical ingredients (API) and excipient in raw or tablet form.
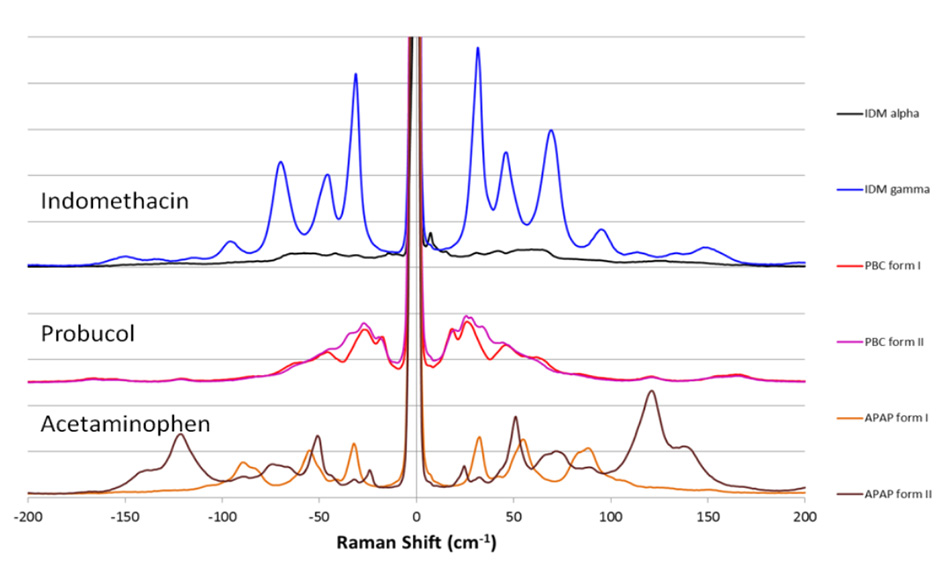
Figure 2: THz-Raman spectra for polymorphs of various APIs showing clear differentiable peaks*.