白皮书
太赫兹拉曼光谱定量分析的制药应用
综述
THz-Raman™ 光谱(也称为低频拉曼光谱)是根据对以下区域范围的振动进行检测的技术:0 至 200 cm-1 范围以及由传统拉曼光谱负责采集的 200 至 1800 cm-1 的化学指纹区域。 该光谱技术最近已成为一种功能强大的定量分析模式,可提供有关结构和相位的实时样品信息,并具有易于使用、遥感和直接测量等全部优势(通常与 NIR 或 FTIR 等光谱技术相关)。 此类结构和相位信息在制药应用中会特别重要,但以前对于此类信息,需要进行繁琐的样品制备和使用较复杂的离线方法,如使用粉末 X 射线衍射 (PXRD) 才可获取。 太赫兹拉曼系统现在可以作为独立仪器使用,或者与现有的传统拉曼仪器集成,以提高其在低波数区域的灵敏度。 可与浸入式探头一起使用,用作样品瓶的台式仪器和显微镜附件,以及集成在孔板读取仪中用于高通量筛选应用。 在制药行业内有着许多应用,本白皮书列举了一些精选的代表性应用,以凸显太赫兹拉曼光谱在制药领域的广泛应用范围

- 多晶型识别
- 共晶体形成
- 与共晶体形成 非晶态监控
- 多种水合物(伪多晶型性)
- 高通量筛选
多晶型识别
许多活性药物成分 (API) 能以多种形式结晶或结晶为多晶型物。 这对于难溶性药物尤其重要,因为各种多晶型物在胃肠道中的溶解特性可能完全不同,影响其生物利用率,从而影响疗效。 过去,许多药物的多晶型性是通过偶然的机会或使用人工且耗时的方法进行检测的。 如今,通过将化学和高通量筛选相结合进行药物晶体工程,可以轻松、全面地识别稳定的多晶型/水合物/无水形式。 这是至关重要的,其意味着在药物制造过程中的开发、制造和质量保证阶段能快速可靠地识别多晶型物。
多晶型物具有相同的分子组成,只是分子在晶格中的排列方式不同。 因此,使用传统的光学分析方法难以识别不同的多晶型物。 例如,FTIR、NIR 和传统的拉曼分析都是基于对光谱峰的测量,光谱峰由指示分子组成的分子内振动引起。 因此,多晶型分析应用通常采用其他方法,例如粉末 X 射线衍射 (PXRD) 或固态核磁共振 (ss-NMR),需要预先准备好采集的样品和复杂的仪器。 已证明,太赫兹拉曼光谱十分适合该应用,因为使用其可以测量样品中的低能振动,此类振动光谱峰主要由于分子间振动产生,例如声子模式和晶格振动。 太赫兹拉曼光谱对晶体形式的细微差异极其敏感。 此外,由于太赫兹拉曼不需要任何特殊的样品制备,可与绿色化学倡议协同作用,旨在减少溶剂的使用。
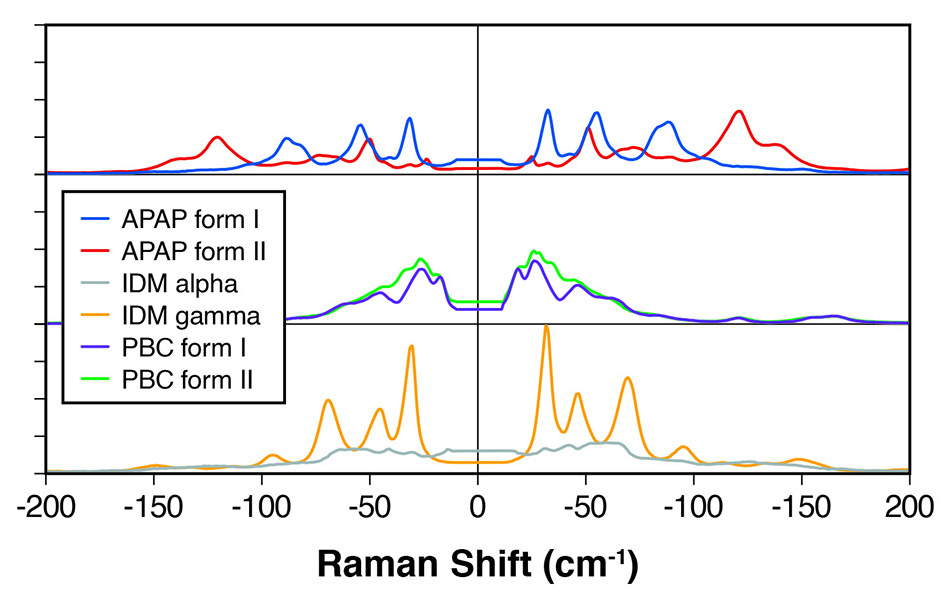
图 1: 用于多种 API 的多晶型物的太赫兹拉曼光谱,显示了明显差异化的光谱峰。
例如,在《Applied Spectroscopy》[1] 的一篇论文中,Coherent(原 Ondax)和 Bristol-Myers Squibb 的科学家证明,太赫兹拉曼分析可成功用于定量区分许多常见 API 的多晶型和非晶态形式,包括卡马西平 (CBZ)、咖啡因、茶碱和阿哌沙班。 他们解释说,太赫兹拉曼光谱对所谓的光学声子进行检测,对于芳香分子结构,其预期频率范围可达约 130 cm-1 (4 THz)。 他们表明,每种多晶型物都具有由晶格模式和某些低频(例如,扭转型)分子内振动组成的特有太赫兹拉曼特征。
在一项相关研究中,Coherent [2] 的科学家证明了其他若干 API 的多晶型物之间存在明显的光谱差异,此类多晶型物包括吲哚美辛、普罗布考和对乙酰氨基酚,如图 1 所示。他们总结说太赫兹拉曼系统可快速、明确地对多晶型物进行区分,与高频拉曼“化学指纹”区域相辅相成,用于化学鉴定。
共晶体的形成
仅对多晶型物进行优化并不总是足以使 API 的配方具有必要的热稳定性、溶解度等。 所以制药业开发了一系列其他方法来改变此类特性。 例如,使用共晶体来调节 API 的可溶度、稳定性和生物利用度。 共晶体是由两个不同分子或离子化合物之间的氢键等弱分子间相互作用形成的分子晶体。 共晶体还与爆炸物、农用化学品和色素关系密切。 在制药领域,主要与低可溶度 API 一起发挥作用。
共晶体由活性药物成分 (API) 和所谓的共成型体组成。 基于不同的化学计量方式,同样的 API/共成型体配对往往可以产生不同的共晶体。 例如,卡马西平:对氨基苯甲酸共晶体系统能以 1:1、2:1 和 4:1 的化学计量组合形式存在,每种组合形式都具有特定的溶解度和其他重要的理化特性。 可通过多种固态、浆液和基于溶液的方法形成共晶体,共晶体制备仍然是一个活跃的研究领域。
因此,从研究到 QA、筛选和过程监控,都需要一种可靠且快速的方法来定量识别特定的共晶体。 传统的光学方法专注于基于共价键的光谱峰,充其量只能显示共晶体和混合物之间以及不同共晶体之间的细微数据差异。 使用粉末 X 射线衍射 (PXRD) 和核磁共振 (NMR) 光谱技术可获取数据,但需要提前将样品采集完备并且使用价格高昂的仪器进行离线测量。 然而,由于太赫兹拉曼光谱是一种主要检测分子间振动和晶格模式的光学方法,非常适合这种分析方面的挑战,如图 2 中的示例所示。
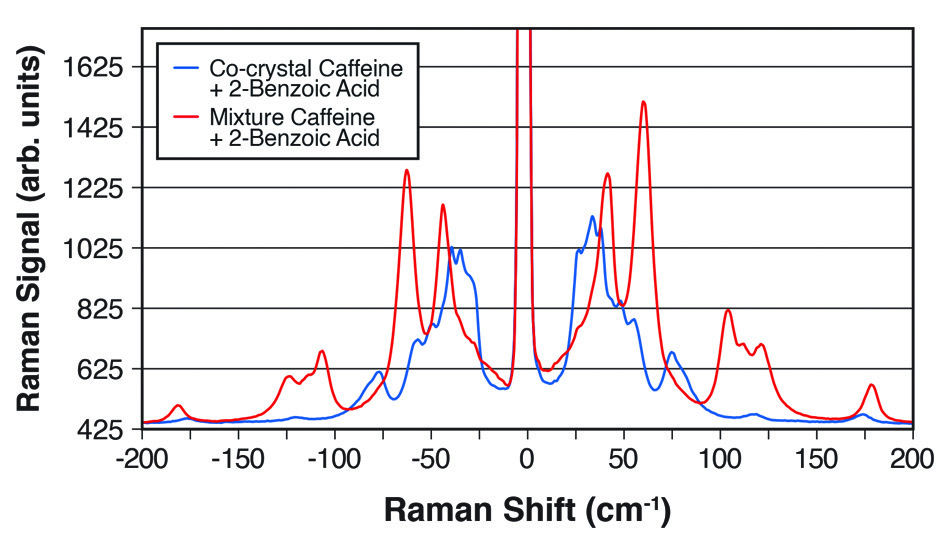
图 2: 太赫兹拉曼光谱显示了咖啡因和 2-苯甲酸的 1:1 共晶体与两种化学物质的简单混合物之间的明显差异。
在《Industrial & Industrial Chemical Research》的一篇论文中 [3],Coherent(原 Ondax)、明治药科大学(东京清濑)和日本国立卫生科学研究所(东京世田谷)的科学家证明,太赫兹拉曼光谱仪可用于定量监控共晶体的形成。 具体来说,他们研究了卡马西平和对氨基苯甲酸通过反应结晶法 (RCM),以乙醇为溶剂,形成的比例为 1:1 和 2:1 的共晶体。 使用光纤耦合至浸入式探头的光谱仪并基于多元曲线解析 (MCR) 进行数据拟合,可实时获得太赫兹拉曼数据。 除了对定量样品进行分析外,他们还表明,该技术可用于跟踪具有不同化学计量方式的共晶体间的转换,并确定反应速率和到达终点的时间,证实了太赫兹拉曼光谱(一个共晶过程中实时且有用的过程分析工具)的效用。
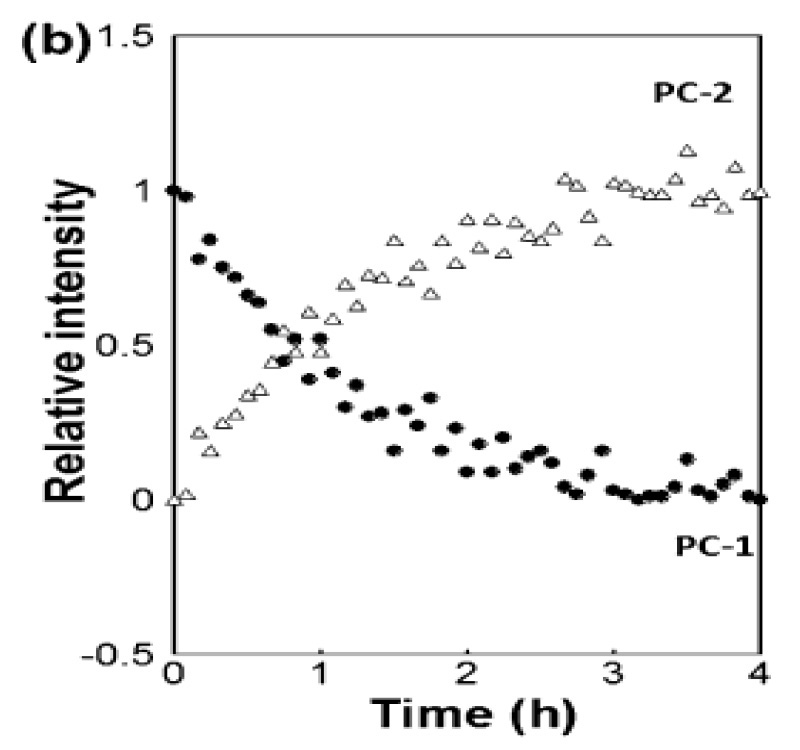
图 3:基于太赫兹拉曼光谱数据的 MCR 拟合,实时监控卡马西平和对氨基苯甲酸构成的比例为 1:1 的共晶体转换为比例为 2:1 的共晶体 经许可转载自 [3]。
结晶度与非晶态监测
低频拉曼光谱以晶格模式和光学声子引发的尖峰为主,这意味着太赫兹拉曼分析仪可用于测量混合相态中特定晶体的浓度,即可充当“结晶度监视器”[4]。主要采用粉末 X 射线衍射 (PXRD) 进行晶体识别,可根据材料的晶格特性,以特定衍射角的亮点图案形式产生数据。 然而,非晶态材料由于其分子的半随机、无序排列而产生大范围衍射 X 射线光晕,这充当了本底噪声并设定了检测限 (LOD)。 这意味着当结晶度含量低于 5% 左右时,使用 PXRD 以可靠的方式测量结晶度含量通常不太可行。
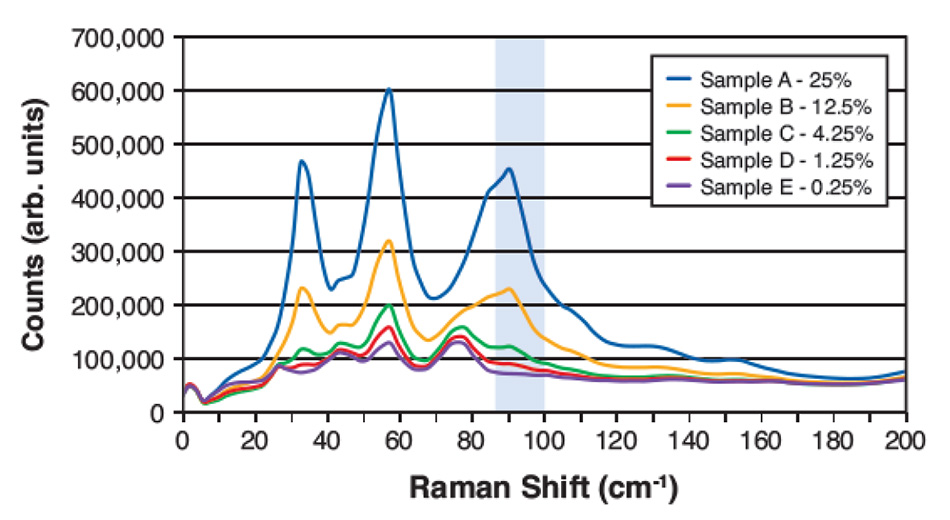
图 4: 太赫兹拉曼光谱,用于测量在甘露醇中的五种不同重量比的对乙酰氨基酚混合物。 高亮区域显示了具有强对乙酰氨基酚信号且与用于计算含量的甘露醇低重叠的区域。
然而,通过太赫兹拉曼光谱,即使在低浓度下,仍然可以明显区分由于晶体成分引发的某些尖峰。 这里的 LOD 取决于选取目标的光谱成分,由于背景材料问题而与光谱特征尽量减少重叠。 图 4 显示了太赫兹拉曼光谱,来自一项最近研究(关于对乙酰氨基酚在甘露醇中的稀释混合物)。 对乙酰氨基酚信号与甘露醇引起的信号的重叠量低,据此选择了高亮频率区域进行拟合。 在考虑到材料间的密度差异并进行背景去除和基线校正后,根据在该光谱窗口上整合的残留信号的数量级,计算出预测的(重量比)浓度。
针对五种不同浓度,使用该简单计算方法得出了计算结果。图 5 显示了计算结果与实际浓度的关系图。 根据该图,确定 LOD 约为 1%,由于样品混合物的局部空间变化,尽管分析区域中光谱特征之间存在一些残留重叠,在低浓度条件下的误差仍然较高。 该 LOD 远远低于 PXRD 的 LOD 典型值,与固态核磁共振 (ss-NMR) 相当。 若将光谱峰间的混合均匀性或分离性较好的材料进行不同组合,LOD 则更低。
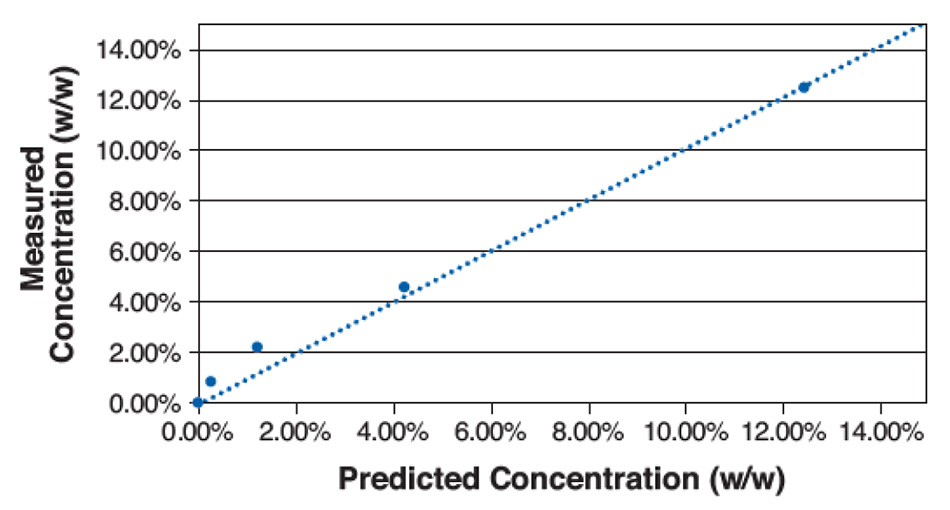
图 5: 甘露醇中多种对乙酰氨基酚混合物的测量浓度与预测浓度的关系图。
Coherent太赫兹拉曼光谱应用实验室其他系统的数据表明,在某些过程监控情况下,即便只进行较简单的分析也能量化结晶度。 具体来说,与非晶态背景的宽玻色子特征相比,晶体峰普遍过于尖锐,通常可以简单地通过分析低频区域的平均光谱导数(斜率)来估算结晶度。
能在实验室和在线实时确定结晶度显然是一个优势,可以广泛适用于制药和化学工业的许多领域。
多种水合物(伪多晶型性)
伪多晶型性是指同一化合物的晶相,因晶格中加入的溶剂材料的数量而不同的现象。 例如,在有含水混合物的情况下,其以半水合相、单水合相、双水合相和三水合相的形式,以及此类混合物的形式出现。 在另一项已发表的研究 [5] 中,Coherent(原 Ondax)、明治药科大学(东京清濑)和日本国立卫生科学研究所(东京世田谷)的科学家证明,太赫兹拉曼光谱能够确定硬脂酸镁 (Mg-St) 固体样品中各种水合物的数量。 他们指出,先前的研究表明,一水合物和二水合物形式的 Mg-St,其粉末润滑性、致密性和流动性均不同。 他们还指出,即便进行了镁盐鉴定并测定了硬脂酸的相对含量(使用气相色谱法)以及水含量,也不能就此唯一确定 Mg-St 的伪多晶型性。 在跨国公司从不同地点采购原材料,并采用不同程度的良好制造规范的情况下,这种不确定性可能会越来越难以捉摸。
在这项研究中,通过使用 PXRD 以及热重和差热分析 (TGDTA) 法对从不同供应商处获得的 10 份 Mg-St 样品进行了分析,从而可以按水合物类型对其进行描述。 高频常规(化学指纹)拉曼光谱显示,水合物之间的差异非常小。 但如图 6 所示,当使用低频区域测量 Mg-St 的各个伪多晶型物时,在 30 至 60 cm−1 范围以及 100 至 180 cm−1 范围内的光谱峰存在差异。 特别是,在 100 至 180 cm-1 的区域内,各水合物的光谱峰明显分离,即便 Mg-St 是伪多晶型混合物,也能区分一水合物和其他水合物。
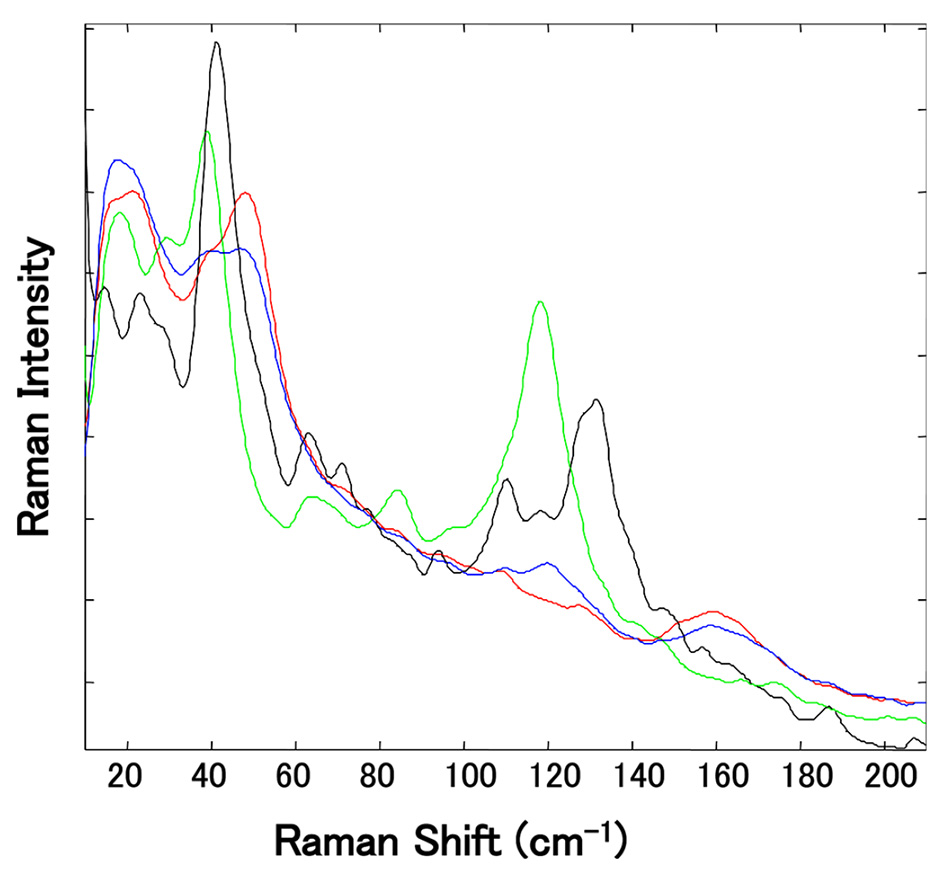
图 6: 若干来源独立的 Mg-St 样品的归一化太赫兹拉曼光谱的放大视图。 (118 cm-1 处出现绿光谱峰是由于存在三水合物,129 cm-1 处出现黑光谱峰则与二水合物有关。) 经许可转载自 [5]。
高通量筛选
上述应用凸显了太赫兹拉曼光谱使用方便,更为简单,可替代 PXRD 和 ss-NMR 用于快速确定样品的均相位信息。 这两种运作不便的老技术都不能进行空间映射。 相比之下,通过物镜可进行拉曼和太赫兹拉曼测量,可在采样区域进行调节(缩放)和扫描,在显微镜下对样品的成分和结构/相态进行唯一映射(成像)。 具有该成像功能,还可以实现孔板映射和孔内映射,适用于高通量筛选 (HTS) 应用。Coherent提供全自动孔板读取器 TR-WPS,可针对 6 至 1536 孔的常见孔板格式进行一站式分析 [6]。
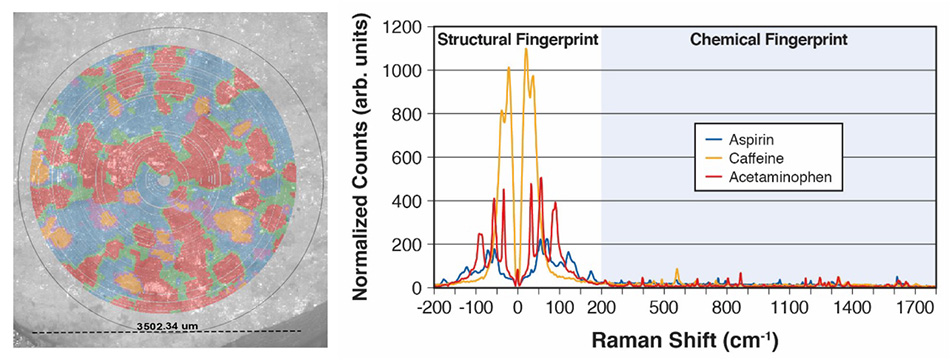
图 7: 埃克塞德林表同时映射了三种 API,以及相应太赫兹拉曼光谱(用于根据主成分分析 (PCA) 和聚类创建该图)。
图 7 显示了假色数据,来自Coherent应用实验室中简单 TR-WPS 演示。 具体而言,将单片 Excedrin® 放置于孔中,并在 9 分钟内在片剂的 3.5 mm 直径区域上收集超过 2.7 万个光谱数据点。 使用主成分分析 (PCA) 和聚类来确定边界区域中的三种成分化合物(对乙酰氨基酚、阿司匹林和咖啡因)以及混合物。 同时使用偏光显微镜 (PLM) 对该区域拍摄 图像,然后覆盖拉曼图以进行视觉比较。 相应光谱清晰地显示了高质量、高数量级数据,以此证明 TR-WPS 能够快速识别和区分材料或多晶型物并且能够捕获视觉和光谱数据。
® Excedrin 是 GlaxoSmithKline 的注册商标
总结
使用太赫兹拉曼光谱可对样品进行实时、非接触式分析,获取结构信息,如果不使用该项技术,则需要通过 PXRD 和 ss-NMR 等运作不便的技术进行样品制备和离线测量。 当其与传统拉曼光谱无缝集成时特别有用,可同时对样品的成分和结构进行检查。 对数项制药业应用的研究表明其潜在用途广泛,它的优势也直接有利于包括聚合物科学、半导体材料和工业化学品在内的许多其他行业。
参考文献
1. P.J. Larkin et al, Polymorph Characterization of Active Pharmaceutical Ingredients (APIs) Using Low-Frequency Raman Spectroscopy, Applied Spectroscopy, Vol 68, Number 7, 2014
2. Coherent, Inc., Polymorph Identification in Pharmaceuticals, Application Highlights, 2019
3. M. Inoue et al, Real-Time Formation Monitoring of Cocrystals with Different Stoichiometries Using Probe-Type Low-Frequency Raman Spectroscopy, Ind. Eng. Chem. Res., Vol, 56, #44, 12693–12697, 2017
4. J. Wallace, Terahertz-Raman Instrument Becomes a Crystallinity Phase Monitor, Laser Focus World, 6, 2020
5. T. Koide et al, Identification of Pseudopolymorphism of Magnesium Stearate by Using Low-Frequency Raman Spectroscopy, Org. Process Res. Dev., Vol 20, 1906−1910, 2016
6. Coherent, Inc., High Throughput Screening Using THz-Raman, Application Highlights, 2020