백서
THz-Raman 분광학 소개
개요
대략 0.15THz에서 6THz까지(5cm-1~200cm-1) 이르는 전자기 스펙트럼의 테라헤르츠(THz) 영역은 물질의 저에너지 진동 모드를 조사하는 수단으로 오랫동안 연구되어 왔습니다. 이 영역은 결정 내의 분자 간 진동을 연구하는 분광기 사용자들에게 특히 매력적이며[1], 물질의 많은 주요 특성을 지배하는 분자 배향에 대한 중요한 정보를 제공합니다. 가장 근본적인 차원에서 보면 THz 분광학은 원적외선(FIR) 흡수 분광학의 확장입니다. 실제로는 광학 주파수 영역의 가장 낮은 끝과 전자 장치용 주파수 영역의 가장 높은 끝에 있기 때문에 구현하기가 훨씬 더 어렵습니다. 그렇기 때문에 출판된 문헌에서 스펙트럼의 이 영역이 "테라헤르츠 갭"으로 불리는 이유입니다[2]. 현재, 상용 THz 분광 시스템은 시중에서 구할 수 있지만 대부분은 THz 방사선을 생성하는 데 일반적으로 필요한 초고속 레이저의 복잡성과 샘플의 습기에 대한 민감성으로 인해 엄청나게 비싸고 사용하기도 어렵습니다.
다행스럽게도 다수가 라만 활성이기 때문에 THz 방사선의 직접적인 흡수가 THz 영역 진동 모드에 액세스하기 위한 유일한 방법은 아닙니다. 라만 산란은 흡수가 아닌 광자의 비탄성 산란으로 인해 발생하므로 조사 중인 진동 모드에서 여기 파장을 분리합니다. 전통적인 라만에서, 200cm-1~1,800cm1 스펙트럼 범위는 대부분의 분자 내 진동이 이 주파수 범위에서 발생하기 때문에 "화학적 지문"을 나타냅니다. 보완적으로, 5cm-1~200cm-1 스펙트럼 범위의 THz-Raman™(저주파 라만 또는 LFR이라고도 함) 영역은 주로 분자 간 진동 또는 물질의 격자/포논 모드에 해당하는 "구조적 지문"을 제공합니다. 그림 1은 THz 분광학(50μm~2mm), IR 분광학(5~50μm) 및 THz-Raman 분광학이 전자기 스펙트럼 내에서 발생하는 상대 파장 위치를 보여줍니다. 그림 2는 그에 상응하는 전형적인 라만 스펙트럼의 화학적 지문 및 구조적 지문의 상대적 위치를 보여 줍니다.
라만분광학은 비탄성 산란(라만 이동)이 탄성 산란(레일리 산란)보다 규모가 훨씬 적어서 레일리 산란을 적절히 필터링하기 어렵게 만들기 때문에 THz 영역에서 구현하는 데 지금까지 어려움을 겪어오고 있습니다. 그 결과, 레일리 라인(0cm-1)에 매우 가까운 라만 이동으로 신호를 분해하기가 어려웠습니다. 높은 광학 효율을 특징으로 하는 단순한 모노크로메이터로 5cm-1의 작은 라만 이동을 직접 측정하기 쉽게 만든 볼륨 홀로그램 노치 필터의 혁명적인 개발이 이러한 상황을 완전히 뒤바꿔 놓았습니다[3]. 이러한 비약적인 발전으로 저주파 라만에 대한 패러다임 전환이 이루어졌습니다. 즉, 복잡한 다단계 모노크로메이터를 필요로 하는 비교적 새로운 것부터 모든 과학자가 불과 몇 분 안에 설정할 수 있는 일체형 분광계 및 기존 기기를 위한 플러그 앤 플레이 추가 기능 모듈에 이르기까지 다양합니다. 예를 들어 이러한 THz-Raman 모듈에는 반응 모니터링을 위한 침지 프로브, 샘플의 위상 매핑을 위한 현미경 플랫폼 및 고처리량 검사 응용 분야를 위한 웰 플레이트 판독기가 포함됩니다.
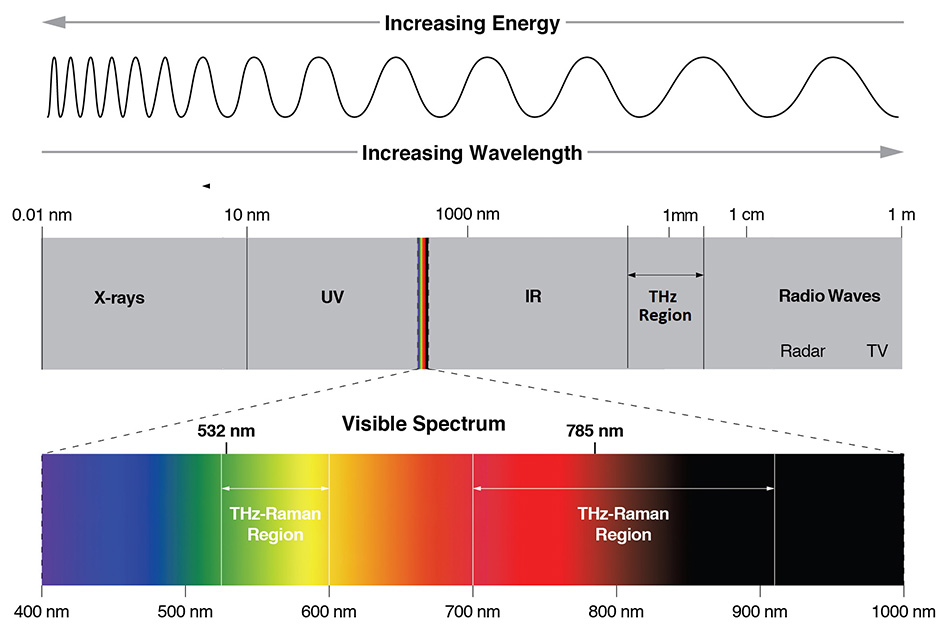
그림 1: 여러 다른 분광 기법의 위치를 보여주는 전자기 스펙트럼 - 라만 분광법은 일반적으로 가시광선(532nm) 또는 NIR(785nm)에서 수행되는 반면 IR 흡수 분광법은 5~50μm에서 THz 분광법은 50μm~2mm에서 수행됩니다.
THz-Raman 시스템 요구 사항
모든 THz-Raman 시스템은 다음과 같은 네 가지 공통점을 가지고 있습니다.
- 파장 안정화 레이저 소스
- 협대역(5cm-1 미만) 스펙트럼 클린업 필터
- 협대역(5cm-1 미만) 레일리 산란 차단 필터
- 저주파 신호를 감지할 수 있는 분광계
레일리 필터는 +/- 5cm1 대역폭 노치 필터로서 시스템이 감지할 수 있는 THz 영역 안에서 얼마나 낮은지를 제한하는 계수여야 합니다. 그러나 레일리 필터가 제대로 작동하려면 레이저의 출력 스펙트럼이 매우 안정적이고 선폭이 매우 좁고 증폭된 자발 방출(ASE) 또는 노치 필터의 전송 영역 내 측파대에서 극도로 낮은 잡음을 나타내는 것이 중요합니다. 또한 레일리 신호의 누출과 분광계의 검출기에 대한 포화를 방지하기 위해 작동 중에 필터 및 레이저의 중심 파장이 서로 "스펙트럼으로 동기화된 상태"를 유지하는 것이 필수입니다. 레이저, 필터 및 광학 시스템의 통합 제조업체인 Coherent의 분광계 및 모듈은 모두 이러한 동기화를 완벽하게 유지하도록 설계되었고 손쉽게 작동할 수 있으며 견고성과 일관된 성능을 보장합니다.
이러한 시스템의 또 다른 장점은 라만 스펙트럼이 레이저 주파수에서 상대적인 이동으로 측정되기 때문에 THz-Raman 시스템은 가시광선 또는 근적외선(NIR)의 모든 파장에서 레이저를 사용하여 이러한 저에너지 진동 모드를 조사할 수 있다는 것입니다. 따라서 THz 레이저 소스가 필요하지 않습니다. 이 덕분에 훨씬 간단하고 비용이 적게 드는 스펙트럼 수집 체계가 가능하므로 유리 광학 및 파이버, 작고 저렴한 다이오드 및 다이오드 펌프 고체 레이저(DPSS) 소스, 실리콘 검출기 및 검출기 어레이를 사용할 수 있습니다. THz-Raman 시스템은 또한 -5cm-1 미만의 반스토크스 라만 이동도 감지할 수 있기 때문에 국소적 유효 온도를 포함해 샘플에 대한 추가 정보를 제공할 수 있습니다. 따라서 THz-Raman 시스템은 단일 측정에서 고속 대량으로 물질의 화학적 지문과 구조적 지문 모두를 동시에 측정할 수 있습니다.
라만 분광학의 사용 편의성과 THz 스펙트럼 정보가 결합됨으로써 이제는 물질의 화학적 및 구조적 특성 모두에 고려할 수 있는 응용 분야를 위한 강력한 솔루션이 되었습니다. THz-Raman은 제약 산업[4~9]에서 빠르게 채택되어 왔으며 폴리머[10], 반도체[11~13] 및 생의학 진단법[14]에서도 주목을 받고 있습니다.
저주파 진동 모드
위에서 설명한 바와 같이 저주파 피크는 포논 모드 및 격자 진동과 같은 분자 간 진동에 의해 발생합니다. 결정질 샘플에서 피크의 위치(이동)는 결정의 구성(화학적 및 구조적) 세부 사항에 따라 다릅니다. 그리고 이러한 뾰족한 저주파 피크의 대역폭 및 강도는 샘플의 구조 정도(결정도)와 직접적인 관련이 있습니다. 그에 반해서, 비정질 고체 및 액체는 보손 피크로 알려져 있는 확인되지 않은 넓은 피크를 가지고 있습니다. 따라서 THz-Raman 스펙트럼은 샘플 결정도를 정량적으로 분석하고 여러 다른 동소체 및 다형체를 분류하는 데 유용한 도구입니다. 다형 분석이 더 많이 알려진 응용 분야이지만, 저주파 진동 모드의 특성을 더 명확하게 나타내기 위해 여러 다른 동소체의 THz-라만 스펙트럼을 조사함으로써 더 쉽게 이해할 수 있습니다.
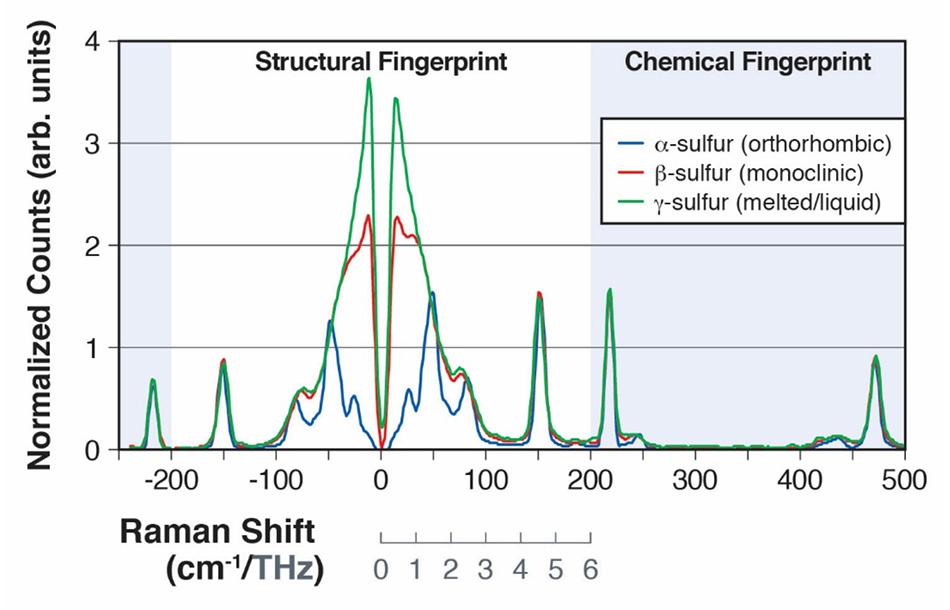
그림 2: 0~200cm-1 사이의 분자 간 진동 모드(A) 및 200cm-1~500 cm-1 사이의 분자 내 진동 모드(B)를 보여주는 α황 및 β황의 라만 스펙트럼
30개 이상의 동소체를 가지고 있는 황은 생명 과학 및 산업 응용 분야 모두에서 광범위한 화학 공정에서의 중요성 때문에 폭넓게 연구되어 왔습니다[15]. 동소체 α황은 24가지 다른 분자 간 진동을 가지고 있는 것으로 나타났으며, 그중 다수는 스펙트럼의 30cm-1~100cm-1 영역에서 라만 활성입니다[16]. 2013 SPIE Defense, Security, and Sensing 컨퍼런스에서 발표된 한 논문에서, Heyler 등은 THz-Raman을 사용하여 이러한 진동 모드를 감지할 수 있는 방법을 설명했습니다[3]. 또한 α황 샘플이 95.6°C 이상으로 가열됨에 따라 동소체 β황으로 형태가 변화된 후 115.2°C에서 액화된다는 것을 보여주었습니다. 보손 피크 구조에 더 가까워짐에 따라 증가된 불규칙성이 100cm-1 미만의 라만 스펙트럼에서 모드를 "흐릿하게" 하기 때문에 α 형태(사방정계)에서 덜 규칙적인 β 형태(단사정계)로의 전이를 쉽게 감지할 수 있습니다[그림 2]. 용해되고 y 형태(액체)를 형성한 후, THz-Raman 영역의 구조 모드가 완전히 혼합되어 일반적인 액체의 전형적인 순수 보손 대역만 표시됩니다[17]. 대조적으로, 화학적 지문 영역의 모드는 100cm-1이상의 스펙트럼의 피크 위치를 비교할 때 명백히 형태 변화에 의해 크게 영향을 받지 않았습니다. 그림 2의 모든 스펙트럼은 직립 현미경 및 파이버 결합 분광계에 부착된 Coherent TR-MICRO THz-Raman을 사용하여 수집되었습니다. 그림 3은 개략적으로 나타낸 구성도입니다.
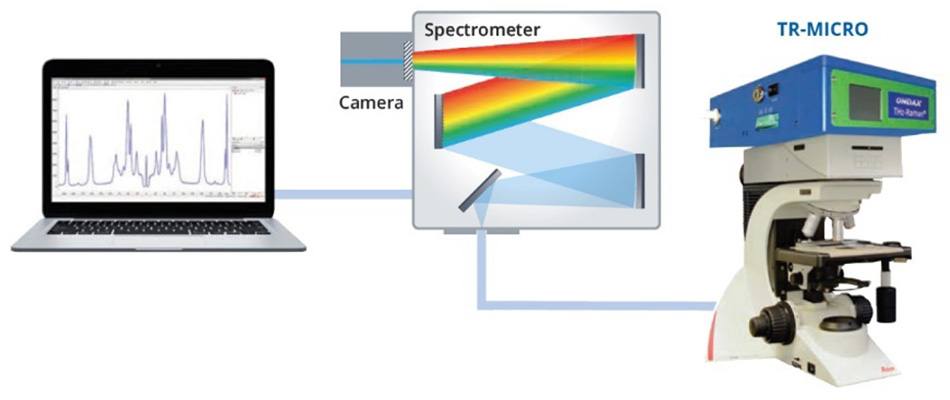
그림 3: α황 및 β황의 THz-Raman 스펙트럼을 수집하는 데 사용된 실험 기구를 개략적으로 나타낸 구성도
THz-Raman 장치에는 초협대역(볼륨 홀로그램) 레이저 라인 및 노치 필터와 정확하게 일치하는 단일 주파수 여기 레이저가 포함되어 있습니다. 노치 필터는 라만 산란의 최대 처리량을 보장하는 동시에 광학 밀도(OD)가 9보다 큰 레일리 산란을 감쇠하도록 특별히 설계되어 불균질 샘플에 사용할 수 있습니다. 마지막으로, 나머지 라만 산란은 파이버 케이블을 통해 분광계에 연결됩니다. 광학 설계에 대한 자세한 설명은 컨퍼런스 자료[3]를 참조하십시오.
의약품에서의 응용
다형성은 활성 제약 성분(API)의 중요하고 공통적인 특성입니다. 이는 원료 의약품 생체 이용률, 제조 가능성 및 품질/성능에 직접적인 영향을 미칩니다[18]. 다형 화합물은 동일한 기본 분자 구성을 가지고 있지만 벌크 구조 배향이 다르기 때문에 다형 형태를 알아내는 데에는 기존의 적외선(IR) 분광학 및 라만 분광학보다 THz-Raman이 훨씬 더 적합합니다. 완벽을 기하기 위해, 경우에 따라 분자 내 진동의 감쇠로 인해 다형 차이가 라만 스펙트럼의 화학적 지문 영역에서 미묘한 피크 이동으로 이어질 수 있다는 점에 유의해야 합니다[19, 20]. 즉, 저주파 범위에서의 스펙트럼 변화는 훨씬 더 뚜렷하고(최대 10배 더 강함) 복잡한 화학 계량 분석 없이도 더 쉽게 구별할 수 있는 경향이 있습니다. 또한 2014년 Applied Spectroscopy에 게재된 논문에서 Larkin 등은 "전형적인 API의 큰 방향족 종의 저주파 라만 스펙트럼은 복잡한 스펙트럼 특징과 함께 200cm-1 미만에서 현저하게 강렬한 대역을 제공한다[9]"고 입증했습니다. 아울러 이러한 대역은 일반적으로 동일한 주파수 범위에서 주변 부형제의 대역보다 훨씬 더 강렬하기 때문에 THz-Raman을 통해 향상된 감도로 "결정질 구조, 결정질 불규칙성 및 비정질 상태"를 직접 측정할 수 있다고 설명했습니다.

그림 4: Coherent의 TR-BENCH(탁상형 THz-Raman 모듈)
앞서 언급한 연구[9]가 발표되고 2015년에 후속 연구[10]가 발표되기 전에 Bristol-Myers Squibb의 연구원들은 일부 일반적인 API의 다형체에 대한 저주파 라만 대역과 관련한 자세한 분석을 제공했습니다. 이 두 간행물에서 연구원들은 인도메타신, 카르바마제핀, 카페인, 테오필린 및 아픽사반을 분석했습니다. Larkin 등은 그림 4에 나와 있는 것처럼 현재 Coherent에서 제공하고 있는 TR-BENCH와 유사한 탁상형 THz-Raman 샘플링 시스템을 사용했습니다. TR-BENCH는 이전 섹션에서 설명한 TR-MICRO와 동일한 내부 광학 아키텍처를 갖추고 있습니다. 이 연구의 한 예로서, 그림 5는 세 가지 다른 형태의 카르바마제핀에 대한 THz-Raman 스펙트럼을 보여 줍니다.
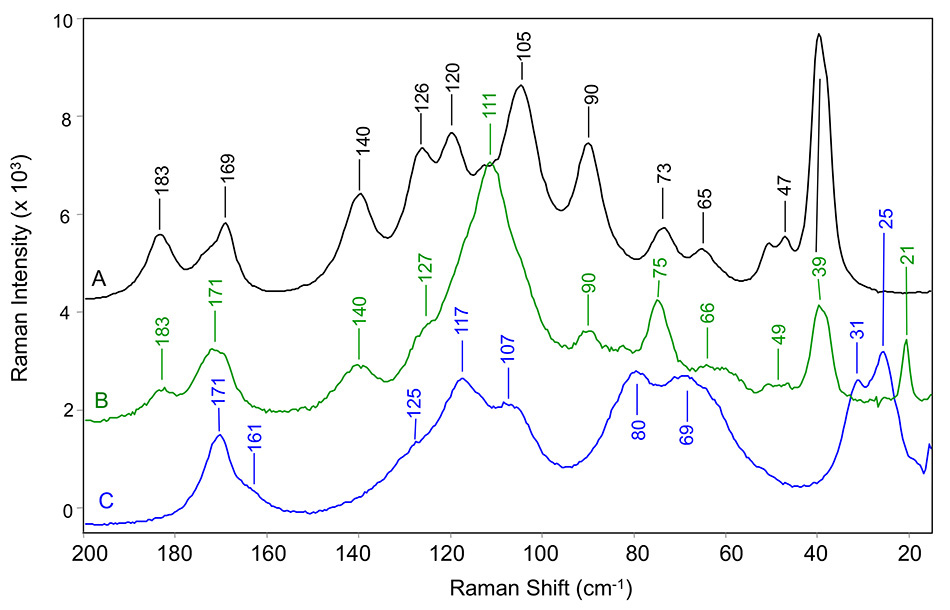
그림 5: (A) 형태 III, (B) 의사 다형 이수화물 형태 및 (C) 형태 II의 카르바마제핀에 대한 THz 라만 스펙트럼 (모두 실온에서 측정됨[8])
최근 몇 년 동안 THz-Raman 분광학은 연구소에서 제약 공정 모니터링(공정 분석 기술(PAT)이라고 함) 응용 분야로 전환되었습니다[21~24]. 공정 응용 분야에서, 파이버로 결합된 THz-Raman 프로브를 사용하면 인라인, 온라인 및 앳라인 측정이 가능합니다. 이 경우 분석기는 샘플로부터 멀리 떨어져 있을 수 있으며 샘플을 채취하여 분석기로 가져올 필요가 없습니다. 요구 사항에 따라 프로브 팁은 접근 포트를 통해 반응 챔버에 직접 담글 수 있도록 짧은 작동 거리로 설계하거나 보기 창을 통해 스펙트럼을 수집할 수 있도록 더 긴 작동 거리로 설계할 수 있습니다. 그림 6은 침지 프로브 팁이 부착된 TR-PROBE를 보여 줍니다.
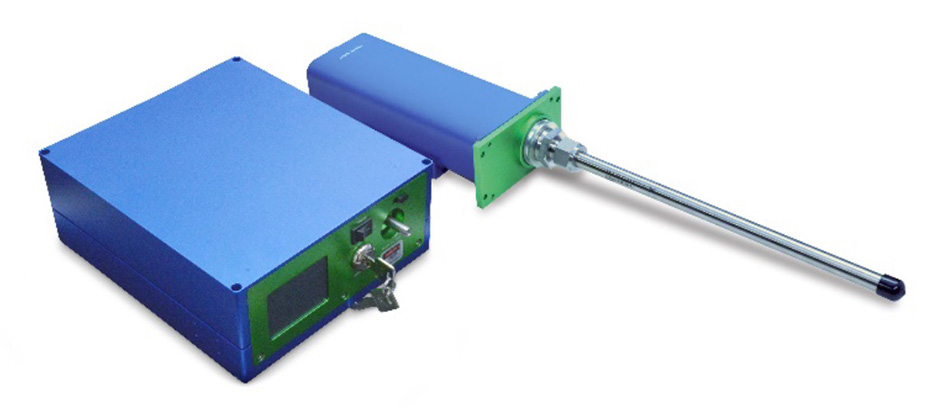
그림 6: 11인치(27.94cm) 길이 스테인리스 스틸 소재의 침지 팁이 부착된 Coherent의 TR-PROBE(THz-Raman 프로브 모듈)
Inoue 등은 다양한 농도의 에탄올과 물에서 카르바마제핀 III형에서 카르바마제핀 이수화물로의 전이를 모니터링하기 위해 이 접근 방식을 사용했습니다[22]. 그림 7에 표시된 결과는 카르바마제핀 이수화물(111 cm-1) 및 카르바마제핀 III(39cm-1)의 지배적인 THz-Raman 대역을 가진 다변량 곡선 분해능(MCR) 알고리즘을 사용하여 계산되었습니다. 이러한 데이터를 기반으로 연구원들은 이 반응의 경우 62.5% 에탄올과 37.5% 물의 용액 사용 시 가장 빨리 변환했음을 알아낼 수 있었습니다.
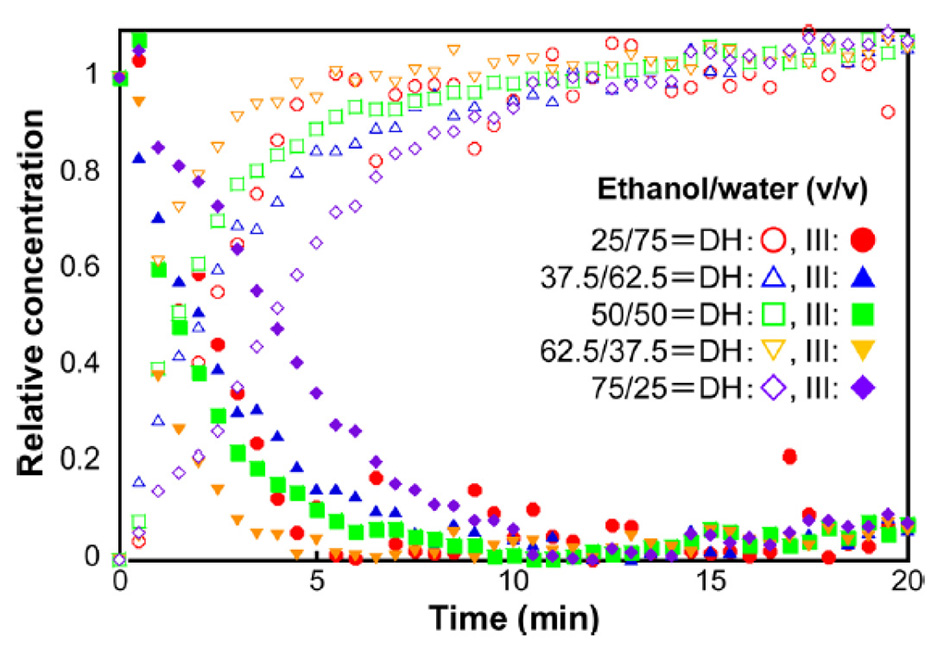
그림 7: 에탄올과 물의 다양한 용제 비율에서 카르바마제핀 III형이 카르바마제핀 이수화물로의 변환에 대한 농도 동역학[21]
THz-Raman의 미래
THz-Raman은 제약 산업에서 처음으로 널리 채택되었으며, 다른 부문에서도 THz-Raman을 사용하여 결정도 및 다형성을 분석하고 있습니다. 한 예로 최근에는 유기 반도체에서 전하 이동 및 저주파 진동[12]뿐만 아니라 이동도 및 변형[25] 간의 관계를 조사하는 데 THz-Raman을 사용하고 있습니다. 또한 양자점[11] 및 층이 있는 반도체 합금[13]에서 포논 모드를 분석하는 데에도 사용되고 있습니다. 또한 폴리머 결정화[10] 및 냉각 과정 중 라멜라 형성[26]에 대한 연구도 최근에 발표되었으며, 이는 제품의 구조적 특성에 대한 중요한 정보를 제공할 수 있어서 폴리머 산업 분야에 유용합니다. 앞으로 THz-Raman의 가장 흥미진진한 응용 분야는 아마도 생물학 및 생의학 진단법일 것입니다. 샌프란시스코에서 열린 2019 SPIE BiOS 컨퍼런스에서 Marble 등은 생물학적 분자에 THz-Raman 사용에 대한 첫 번째 강연을 선보였으며[14], 그리고 1년 후에 THz-Raman은 이미 COVID-19에 대한 잠재적 진단 도구로서 연구되고 있었습니다[27].
요약
THz-Raman은 사용자에게 명확한 구조적 및 화학적 구성 정보를 동시에 제공할 수 있는 것으로 거듭 증명되고 있습니다. THz-Raman 분석기가 실험실에서 산업 환경으로 이전됨에 따라 THz-Raman 응용 분야가 계속해서 성장하고 확장될 것이라는 데는 의심의 여지가 없습니다. 이 시점에서 연구원들은 THz-Raman에 의해 밝혀진 고도로 차별화된 정보에 대해 아직 구상되지 않은 새로운 용도를 계속해서 찾아내야 할 것입니다. THz-Raman 계측, 응용 분야 또는 기능에 대한 자세한 내용은 www.thz-raman.com을 방문하거나 당사 웹사이트 www.coherent.com을 통해 응용 과학자와의 상담을 요청하십시오.
참고 문헌:
[1] El Haddad, J., Bousquet, B., Canioni, L. and Mounaix, P., 2013. Review in terahertz spectral analysis. TrAC Trends in Analytical Chemistry, 44, pp.98-105.
[2] Dexheimer, S.L. ed., 2017. Terahertz spectroscopy: principles and applications. CRC Press
[3] Heyler, R.A., Carriere, J.T. and Havermeyer, F., 2013, May. THz-Raman: accessing molecular structure with Raman spectroscopy for enhanced chemical identification, analysis, and monitoring. In Next-Generation Spectroscopic Technologies VI(Vol. 8726, p. 87260J). International Society for Optics and Photonics
[4] Gato, K., Fujii, M.Y., Hisada, H., Carriere, J., Koide, T. and Fukami, T., 2020. Molecular state evaluation of active pharmaceutical ingredients in adhesive patches for transdermal drug delivery. Journal of Drug Delivery Science and Technology, p. 101800
[5] Koide, T., Fukami, T., Hisada, H., Inoue, M., Carriere, J., Heyler, R., Katori, N., Okuda, H. and Goda, Y., 2016. Identification of pseudopolymorphism of magnesium stearate by using low-frequency Raman spectroscopy. Organic Process Research & Development, 20(11), pp. 1906~1910
[6] Tanabe, Y., Maeno, Y., Ohashi, K., Hisada, H., Roy, A., Carriere, J., Heyler, R. and Fukami, T., 2019. Screening a trace amount of pharmaceutical cocrystals by using an enhanced nano-spot method. European Journal of Pharmaceutics and Biopharmaceutics, 136, pp. 131~137
[7] Larkin, P.J., Wasylyk, J. and Raglione, M., 2015. Application of low-and mid-frequency Raman spectroscopy to characterize the amorphous-crystalline transformation of indomethacin. Applied Spectroscopy, 69(11), pp. 1217~1228
[8] Larkin, P.J., Dabros, M., Sarsfield, B., Chan, E., Carriere, J.T. and Smith, B.C., 2014. Polymorph characterization of active pharmaceutical ingredients (APIs) using low-frequency Raman spectroscopy. Applied Spectroscopy, 68(7), pp. 758~776
[9] Walker, G., Römann, P., Poller, B., Löbmann, K., Grohganz, H., Rooney, J.S., Huff, G.S., Smith, G.P., Rades, T., Gordon, K.C. and Strachan, C.J., 2017. Probing pharmaceutical mixtures during milling: The potency of low-frequency Raman spectroscopy in identifying disorder. Molecular Pharmaceutics, 14(12), pp. 4675~4684
[10] Marlina, D., Hoshina, H., Ozaki, Y. and Sato, H., 2019. Crystallization and crystalline dynamics of poly (3-hydroxybutyrate)/poly (4-vinylphenol) polymer blends studied by low-frequency vibrational spectroscopy. Polymer, 181, p. 121790
[11] Mork, A.J., Lee, E.M., Dahod, N.S., Willard, A.P. and Tisdale, W.A., 2016. Modulation of low-frequency acoustic vibrations in semiconductor nanocrystals through choice of surface ligand. The journal of physical chemistry letters, 7(20), pp. 4213~4216
[12] Sosorev, A.Y., Maslennikov, D.R., Kharlanov, O.G., Chernyshov, I.Y., Bruevich, V.V. and Paraschuk, D.Y., 2019. Impact of Low‐Frequency Vibrations on Charge Transport in High‐Mobility Organic Semiconductors. physica status solidi (RRL)–Rapid Research Letters, 13(3), p.1800485.
[13] Tharith, S., Hai, N.T.M., Lee, Y., Lim, S.Y., Van Quang, N., Kwanpyo, K., Sunglae, C. and Hyeonsik, C., 2020. Optical phonons of SnSe (1− x) S x layered semiconductor alloys. Scientific Reports(Nature Publisher Group), 10(1)
[14] Marble, K.S., Noojin, G.D., Coker, Z.N., Lalonde, J.W., Denton, M.L., Echchgadda, I., Yakovlev, V.V. and Cantu, J., 2019, March. Implementing low-frequency Raman spectroscopy to study biological molecules (Conference Presentation). In Optical Interactions with Tissue and Cells XXX (Vol. 10876, p. 108760H). International Society for Optics and Photonics
[15] Steudel, R. and Eckert, B., 2003. Solid sulfur allotropes. In Elemental sulfur and sulfur-rich compounds I (pp. 1-80). Springer Berlin Heidelberg
[16] Eckert, B. and Steudel, R., 2003. Molecular spectra of sulfur molecules and solid sulfur allotropes. In Elemental Sulfur und Sulfur-Rich Compounds II (pp. 31-98). Springer Berlin Heidelberg
[17] Raw, A.S., Furness, M.S., Gill, D.S., Adams, R.C., Holcombe Jr, F.O. and Lawrence, X.Y., 2004. Regulatory considerations of pharmaceutical solid polymorphism in Abbreviated New Drug Applications (ANDAs). Advanced drug delivery reviews, 56(3), pp.397-414.
[18] Bras, L.P. and LOUREIRO, R., 2013. Polymorphic conversion monitoring using real-time Raman spectroscopy. Chimica Oggi-Chemistry Today, 31, p. 5
[19] Mazivila, S.J., Nogueira, H.I., Páscoa, R.N., Ribeiro, D.S., Santos, J.L., Leitão, J.M. and da Silva, J.C.E., 2020. Portable and benchtop Raman spectrometers coupled to cluster analysis to identify quinine sulfate polymorphs in solid dosage forms and antimalarial drug quantification in solution by AuNPs-SERS with MCR-ALS. Analytical Methods, 12(18), pp.2407-2421.
[20] Otaki, T., Tanabe, Y., Kojima, T., Miura, M., Ikeda, Y., Koide, T. and Fukami, T., 2018. In situ monitoring of cocrystals in formulation development using low-frequency Raman spectroscopy. International journal of pharmaceutics, 542(1-2), pp. 56~65
[21] Inoue, M., Hisada, H., Koide, T., Carriere, J., Heyler, R. and Fukami, T., 2017. In situ monitoring of crystalline transformation of carbamazepine using probe-type low-frequency Raman spectroscopy. Organic Process Research & Development, 21(2), pp.262-265.
[22] Nomura, K., Titapiwatanakun, V., Hisada, H., Koide, T. and Fukami, T., 2020. In situ monitoring of the crystalline state of active pharmaceutical ingredients during high-shear wet granulation using a low-frequency Raman probe. European Journal of Pharmaceutics and Biopharmaceutics, 147, pp. 1~9
[23] Walker, G., Römann, P., Poller, B., Löbmann, K., Grohganz, H., Rooney, J.S., Huff, G.S., Smith, G.P., Rades, T., Gordon, K.C. and Strachan, C.J., 2017. Probing pharmaceutical mixtures during milling: The potency of low-frequency Raman spectroscopy in identifying disorder. Molecular Pharmaceutics, 14(12), pp.4675-4684.
[24] Choi, H.H., Yi, H.T., Tsurumi, J., Kim, J.J., Briseno, A.L., Watanabe, S., Takeya, J., Cho, K. and Podzorov, V., 2020. A Large Anisotropic Enhancement of the Charge Carrier Mobility of Flexible Organic Transistors with Strain: A Hall Effect and Raman Study. Advanced Science, 7(1), p. 1901824
[25] Samuel, A.Z. and Hamaguchi, H.O., 2018. A General Approach for Estimating Lamella‐Thickness Distribution in Polymers with Low‐Frequency Raman Spectroscopy: Application to Lamella Formation in Crystallizing Polyethylene. Chemistry–A European Journal, 24(37), pp.9333-9339.
[26] Damle, V.H., Rajeswaran, B. and Tischler, Y.R., 2020. Low-Frequency Raman Spectroscopy as a Diagnostic Tool for COVID-19 and other Coronaviruses. Royal Society Open Science (In Review).